
Context: India conducts Covid-19 vaccination dry run.
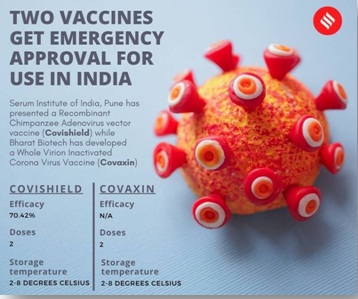 Background: The approval of Drug regulator to Cadila Healthcare Ltd to conduct Phase 3 clinical trials of its ZyCoV-D vaccine candidate for Covid-19. India’s fight vaccine against Covid-19, which has infected more than 1 crore people and killed 1.5 lakh, received a green signal from the Drug Controller General of India (DCGI), the country’s national drug regulator, approved two coronavirus vaccines for restricted emergency use. Serum Institute of India’s Covishield (the Indian variant of the AZD1222 vaccine developed by Oxford University and AstraZeneca) and Bharat Biotech’s Covaxin.
Background: The approval of Drug regulator to Cadila Healthcare Ltd to conduct Phase 3 clinical trials of its ZyCoV-D vaccine candidate for Covid-19. India’s fight vaccine against Covid-19, which has infected more than 1 crore people and killed 1.5 lakh, received a green signal from the Drug Controller General of India (DCGI), the country’s national drug regulator, approved two coronavirus vaccines for restricted emergency use. Serum Institute of India’s Covishield (the Indian variant of the AZD1222 vaccine developed by Oxford University and AstraZeneca) and Bharat Biotech’s Covaxin.
On safety concerns regarding the vaccines, Drugs Controller General of India, said, that It will never be approved, anything if there’s slightest of safety concern. Vaccines are 110 % safe. Some side effects like mild fever, pain & allergy are common for every vaccine.
Besides, the DCGI also gave the green signal to Cadila Healthcare Ltd to conduct Phase 3 clinical trials of its ZyCoV-D vaccine candidate for Covid-19. The Pfizer vaccine and Russia’s Sputnik-V are likely to be approved for use in India in the coming weeks. The government is now set to embark on one of the largest immunisation programmes, with nearly 3 crore healthcare and frontline workers set to be dosed in the first phase.
India: Global Player
India on Sunday became the fourth country after the UK, Argentina and El Salvador to approve the coronavirus vaccine developed by Oxford University and AstraZeneca, which is being manufactured in the country by Pune-based Serum Institute. SII has already stockpiled more than 50 million doses of Covishield so far and currently has a capacity to make around 50-60 million doses a month.
The Effectiveness of the AstraZeneca/Oxford vaccine was found to be 70.42 per cent well below vaccines from Pfizer and Moderna, but above the 50 per cent threshold set by many regulators.
The firm submitted safety, immunogenicity and efficacy data generated on 23,745 participants aged 18 years or older from overseas clinical studies. Data of Phase-2/3 clinical trials on 1,000 participants within the country was also submitted and it was found comparable with the data from the overseas clinical studies.
Facts & Figures
The vaccine, however, has been plagued by uncertainty about its most effective dosage ever since data published in November showed a half dose followed by a full dose had a 90 per cent success rate while two full shots were 62 per cent effective. In December, citing data from early trials, Oxford said the vaccine had a better immune response when a two full-dose regime was given.
Dosage, protection duration and storage: The Subject Expert Committee (SEC) has recommended the approval of two full doses of the vaccine administered around 4-6 weeks apart. Immune response could last at least a year. The vaccine can be stored at temperatures between 2°C and 8°C.
Covaxin: India’s Biotech
Covaxin has been indigenously developed by Hyderabad-based Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR).
Type of vaccine: Covaxin is an inactivated vaccine and is made by using particles of the coronavirus that were killed, making them unable to infect or replicate. Injecting particular doses of these particles serves to build immunity by helping the body create antibodies against the dead virus.
Its effectiveness can be detected by the fact that, it is safe and provides a robust immune response, stated by the Drug Controller General f India. The vaccine is yet to complete late-stage human clinical trials in India and no efficacy rate has yet been made public.
Phase 1 and Phase 2 clinical trials were conducted on about 800 subjects and the results have demonstrated that the vaccine is safe and provides a robust immune response. The Phase 3 efficacy trial was initiated in India on 25,800 volunteers and till date, approximately 22,500 participants have been vaccinated across the country and the vaccine has been found to be safe.
Dosage, protection duration and storage: The vaccine will be administered in two doses and stored at 2-8° degrees Celsius. While the DCGI has not clarified the intervals between the shots, Bharat Biotech had earlier said the efficacy is to be determined only after 14 days post the second dose.
Connecting the Article
Question for Prelims : Consider the following statements regarding Covaxin:
1.Covaxin has been indigenously developed by Hyderabad-based Bharat Biotech.
2.It is an “inactivated” vaccine and is made by using particles of the coronavirus.
Which of the statements given above is/are correct?
(a) 1 only
(b) 2 only
(c) Both 1 and 2
(d) Neither 1 nor 2
Question for Mains : India became the fourth nation after the UK, Argentina and El Salvador to approve the coronavirus vaccine developed by Oxford University and AstraZeneca, which is being manufactured in the country by Pune-based Serum Institute. Suggest the measures for the effective implementation of the vaccination programme.

Our support team will be happy to assist you!
